Abstract
Introduction
Venous thromboembolism (VTE) is the 2nd most common hospital acquired condition at our pediatric hospital. Children with cancer and blood disorders have acquired prothrombotic risk factors. As part of a quality improvement (QI) initiative to decrease VTE in hospitalized children with cancer and blood disorders, we implemented VTE risk assessment, prevention, and documentation tools within the Electronic Health Record (EHR). The baseline documented screening prior to the tool was 0% and sequential compression device (SCD) and prophylactic anticoagulation were rarely prescribed. The primary outcome was percentage of patients with documented screening.
Methods
A VTE risk assessment tool, including the Braden Q activity score (score <3 is defined as chair or bedridden) and clinical risk factors, was developed based on published literature, clinical practice guidelines, local experience, and recommendations from the Children's Hospitals' Solutions for Patient Safety. Patients were categorized as LOW, MODERATE, or HIGH risk for VTE and risk-based recommendations for VTE prevention were provided. MODERATE risk was defined as Braden Q activity score <3 plus 0-1 other risk factors; SCD were recommended if not contraindicated. HIGH risk was defined as central venous catheter (CVC) in place and/or Braden Q activity score <3 plus 2 or more other risk factors; SCD and prophylactic anticoagulation were recommended if no contraindications. Patients are eligible for risk assessment if they are 10-17.99 years and admitted to the hematology/oncology unit at Rady Children's Hospital San Diego for >24 hours.
Results
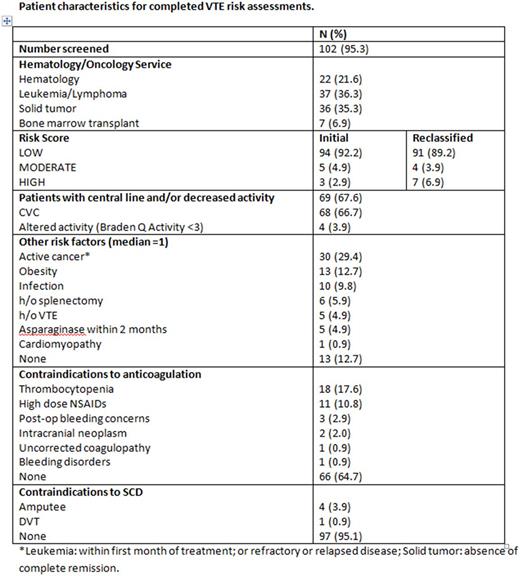
Between March 1 to May 31, 2017, 57 unique patients had a total of 107 admissions, and VTE risk assessment was completed for 102 admissions (95.3%), see Table 1. Members of the VTE Prevention Taskforce reviewed the accuracy of the risk assessments. Four LOW risk patients were reclassified as HIGH risk and 1 MODERATE risk patient was reclassified as HIGH risk. One HIGH risk patients with new diagnosis of VTE had therapeutic anticoagulation ordered and SCD placed on the unaffected lower extremity. Three MODERATE risk patients had SCD placed. Two patients who were initially classified as LOW risk during hospitalization were subsequently diagnosed with CVC related upper extremity deep vein thrombosis (DVT) after hospital discharge. New risk factors at the time of DVT diagnosis were obesity in one patient and recent asparaginase treatment in the other.
Tools which positively impacted adherence with the risk assessment are a Best Practice Advisory (BPA) which fired for medical providers when an eligible patient was admitted, a BPA which fired once the risk category was assigned, a VTE Registry which included all eligible patients, and a VTE Quality Measure Screen which the VTE Prevention Champion used to identify patients who had not been screened and those who were identified at HIGH and MODERATE risk. Medical decision making was documented with a VTE risk assessment note located in the Admission Navigator.
Conclusions
We effectively implemented VTE risk assessment, prevention, and documentation tools in the EHR. Additional QI cycles are required to determine the optimal VTE risk assessment, to improve accuracy of screening, and to increase adherence with recommended interventions to decrease VTE.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal